
Baroness Merron, the Department of Health and Social Care, has provided the following answer to your written parliamentary question (HL2089):
Question by Lord Alton of Liverpool:
To ask His Majesty’s Government how many Yellow Card reports of sexual dysfunction and persistent sexual dysfunction the MHRA received for bupropion in each year since 2014; and what consideration NHS England has given to adding sexual dysfunction as a side effect on the patient information leaflets for bupropion. (HL2089)
Tabled on: 29 October 2024
Answer:
Baroness Merron:
In the United Kingdom there are two authorised products containing bupropion. The first contains bupropion hydrochloride and is prescribed to help individuals stop smoking, when they also have motivational support, for instance through a stop smoking programme. The second is a combination product containing bupropion hydrochloride and naltrexone hydrochloride, and is prescribed in obese or overweight adults to manage weight, together with a reduced calorie diet and physical exercise.
The Medicines and Healthcare products Regulatory Agency (MHRA) has received three Yellow Card reports of sexual dysfunction related reactions suspected to be associated with bupropion hydrochloride, the single constituent, and one Yellow Card report of sexual dysfunction for the combination product, bupropion hydrochloride and naltrexone hydrochloride, between 1 January 2014 and 29 October 2024.
The following table shows the number of suspected Yellow Card reports of sexual dysfunction related reactions and bupropion containing products received by the MHRA between 2014 and 2024:
| Year | Reports of sexual dysfunction single constituent bupropion | Reports of sexual dysfunction combination product bupropion and naltrexone |
| 2014 | 0 | 0 |
| 2015 | 0 | 0 |
| 2016 | 0 | 0 |
| 2017 | 0 | 0 |
| 2018 | 0 | 0 |
| 2019 | 0 | 0 |
| 2020 | 1 | 1 |
| 2021 | 0 | 0 |
| 2022 | 1 | 0 |
| 2023 | 0 | 0 |
| 2024 | 1 | 0 |
Source: data provided by the MHRA.
Note: the data includes reactions grouped under the Medical Dictionary for Regulatory Activities’ (MedDRA) Higher Level Terms: erection and ejaculation conditions and disorders; orgasmic disorders and disturbances; sexual and gender identity disorders NEC; sexual arousal disorders; sexual desire disorders; sexual dysfunction NEC; sexual function and fertility disorders NEC; and spermatogenesis and semen disorders.
Persistent sexual dysfunction does not represent a specific medical condition, so this precise term is not a category available to undertake a structured search of the MHRA’s Adverse Drug Reaction database, and would rely on manual assessment of individual cases. The structured data field search terms are drawn from the regulatory drugs dictionary, MedDRA, or from terms adopted in clinical coding guidance such as the Diagnostic and Statistical Manual of Mental Disorders or the International Classification of Diseases.
It is important to note that the inclusion of a particular report on the MHRA’s system does not necessarily mean that the adverse reactions reported have been caused by the suspect drug. Additionally, the number of reports received should not be used as a basis for determining the incidence of a reaction, as neither the total number of reactions occurring, nor the number of patients using the drug, is known.
The product information, which includes the patient information leaflet, for single constituent bupropion reflects the data currently available, and does not include sexual dysfunction as a possible side effect. The product information for the combination bupropion and naltrexone product contains the terms loss of libido, libido disorder, and erectile dysfunction. As with other medicines, the safety of bupropion is kept under review by the MHRA, and consideration will be given to any emerging evidence on this issue.
Date and time of answer: 08 Nov 2024 at 10:16.
===
Baroness Merron, the Department of Health and Social Care, has provided the following answer to your written parliamentary question (HL2088):
Question by Lord Alton of Liverpool :
To ask His Majesty’s Government how many prescriptions for bupropion were issued in each year since 2014; and what was the annual cost to the National Health Service of those prescriptions for each of those years. (HL2088)
Tabled on: 29 October 2024
Answer:
Baroness Merron:
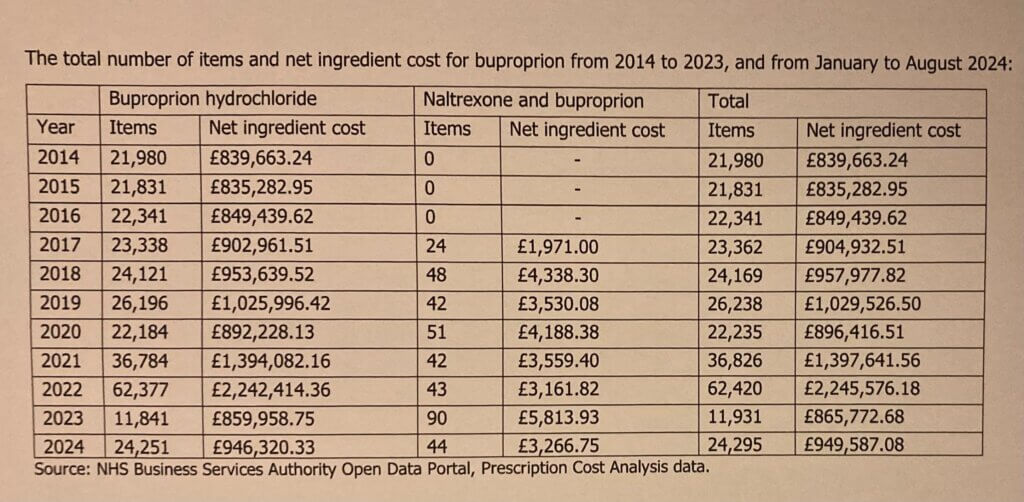
The attached table shows the number of prescriptions issued for buproprion and the net ingredient cost (NIC) from 2014 to 2023, and from January to August for 2024, as this is the latest data available.
Based on the information within the Prescription Cost Analysis published statistics from the NHS Business Services Authority, there are two chemical formulations that include buproprion, those being: bupropion hydrochloride; and the combination of naltrexone and buproprion. It should also be noted that the total NIC shown in the attached table is the basic price of the prescribed medicine before discounts, dispensing costs, or fees. This includes items that have been dispensed in the community in England regardless of where prescribed.
The following documents were submitted as part of the answer and are appended to this email:
Date and time of answer: 08 Nov 2024 at 10:14.

