Primodos: A Welcome Review Into A Drug That Did Enormous Harm
House of Lords February 28th 2019
Letter from Baroness Blackwood to Lord Alton
HPT studies_quality assessment
2.31 pm
Lord Alton of Liverpool (CB)
My Lords, I join others in congratulating my noble friend Lord Carrington on his very well-judged maiden speech today.
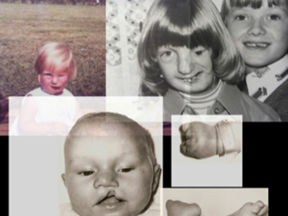
In this welcome debate, my remarks will centre on Primodos—an issue I raised with the noble Lord, Lord O’Shaughnessy, while he was a Minister.
Like the noble Lord, I pay tribute to Marie Lyon, who chairs the Association for Children Damaged by Hormone Pregnancy Tests. Assiduously and tenaciously, she has fought for justice for those whom big pharmaceuticals have often treated with irresponsible contempt. She and her husband have travelled down from Wigan today and are watching our debate.
Marie Lyon wishes me to thank the noble Baroness, Lady Cumberlege, and the Independent Medicines and Medical Devices Safety Review for taking the campaigners seriously. She tells me:
“The sensitivity shown to our members by the”,
review,
“team is appreciated and commended. I really do feel that”,
they and,
“Baroness Cumberlege … are committed to discovering the truth about the failures of the Drug Company and the Government Regulators and have a genuine desire to ensure justice is served”.
I add my own thanks to the noble Lord, Lord O’Shaughnessy, for his role in encouraging the establishment of the independent review, and I wholly endorse what he said earlier about the desirability of creating a national office for patient safety.
My interest in Primodos began in 2010, when a gentleman born with severe birth defects asked to see me at my university office in Liverpool.
He believed that his disabilities were attributable to Primodos, a hormone-based pregnancy test first marketed in the UK in 1959 and produced by Schering AG, which was subsequently taken over by Bayer AG.
Withdrawn from sale in the United Kingdom in 1978, tellingly it was also used in South Korea to abort the child in the womb.
Dr Isabel Gal’s 1960 research at Queen Mary’s Hospital for Children demonstrated a link between the drug and severe birth defects, and a review by the Committee on Safety of Medicines concluded that pregnant women should not use it. However, subsequent court cases failed to provide a conclusive outcome, as did a 2014 review by the Medicines and Healthcare products Regulatory Agency.
On 26 October 2010, I asked the Government several Questions.
One was about the dosage of the constituents of Primodos; one asked for any documents that the Government held about its dangers to be placed in the Library; one was about the nature of the disabilities; one was about the help given to those affected; and one requested Ministers to meet the Association for Children Damaged by Hormone Pregnancy Tests.
In his reply at the time, the noble Earl, Lord Howe, said that the regulatory agency had no information on the number of children who are born with disabilities, nor did it have evidence.
If there was no evidence, why did they ban the drug? As for meeting the victims:
“The MHRA therefore has no current plans to meet members of the Association for Children Damaged by Hormone Pregnancy Tests, people suspected to have been adversely affected by the drug Primodos, or with the pharmaceutical company, Bayer”.—[Official Report, 26/10/10; col. WA 265.]
Despite further letters and Questions, a 2017 report of an expert working group of the UK Commission on Human Medicines continued to state that there was no causal association. Yet in that same year, Sky News broadcast “The Secret Drug Scandal”, which found that evidence of an association had been destroyed by a UK regulator in the 1970s. I asked the Government for their response and,
“whether they will consider establishing a public inquiry into the alleged failure of the regulator at that time to protect public safety”.
In another Question, I asked whether they would examine why,
“no toxicology or testing was undertaken prior to the drug Primodos being licensed”,
and whether they were aware that,
“Primodos was being used as an abortifacient in some parts of the world whilst being sold in the UK for the purposes of pregnancy testing, and … that there may have been collusion between the drug manufacturer and the regulatory bodies”.
In another, I asked why Primodos had stayed on the market and no tests had been,
“ordered by the Committee for the Safety of Medicines under the Medicines Act 1971”.
In another, I asked them to,
“meet with Marie Lyon and representatives of the Primodos victims support group”,
and in another, asked why they were not funding research in Aberdeen and Cambridge examining the,
“likely effects on the child in the womb”.
Then, in February 2018, the right honourable Jeremy Hunt announced his welcome review to be led by the noble Baroness, Lady Cumberlege.
I hope that when the Minister replies, she will tell us when it is likely to report and—perhaps more importantly—who will be responsible for taking forward its recommendations.
Among other things, as we heard from the noble Baroness, the review will investigate any association between hormone pregnancy tests and their teratogenic effects, and whether the regulatory bodies could, and should, have acted on concerns sooner—and if they did not, why.
Meanwhile, a team at Oxford, led by Professor Carl Heneghan, the scientist responsible for identifying Thalidomide association, has discovered that pooled data show “a clear association” with several forms of malformation.
Professor Neil Vargesson has carried out other work on zebrafish, which revealed anomalies that mirrored the adverse effects on victims of Primodos. Their studies were peer-reviewed and remain in the top percentile of scientific studies.
In the House of Commons, the Prime Minister said:
“Ministers are aware of the new study that has come out … and … that study will be looked at very carefully”.—[Official Report, Commons, 16/1/19; col. 1160.]
I welcome that.
However, the raw data that Professor Heneghan needs to complete his review has not been made available. The All-Party Parliamentary Group on Hormone Pregnancy Tests, chaired by Yasmin Qureshi MP, and of which I am vice-chairman, has sent a freedom of information request for the data, but to date has not received a response.
Mrs Lyon has twice emailed the Medicines and Healthcare products Regulatory Agency, but has not received a response. I gave the Minister notice of my intention to raise this question today.
This is tardy and unco-operative on the part of that body. I hope the Minister will be able tell us whether more can be done to take that forward.
Severely disabled children, cared for by family members now in their late 70s, are increasingly becoming the responsibility of their siblings.
While their health deteriorates, many battle every day to support themselves. Some have died fighting to the very end to reveal the truth about the failures of the drugs company and the regulatory agencies.
They have faced the implacable determination of regulatory bodies spending huge amounts of public money on ad hoc scientific reviews to cast doubt on the work of highly reputable scientists. Those who have suffered so grievously deserve much better than this.
Also see:

